HCOOCH CH2 H2O Explained: How a Simple Formula Could Shape the Future
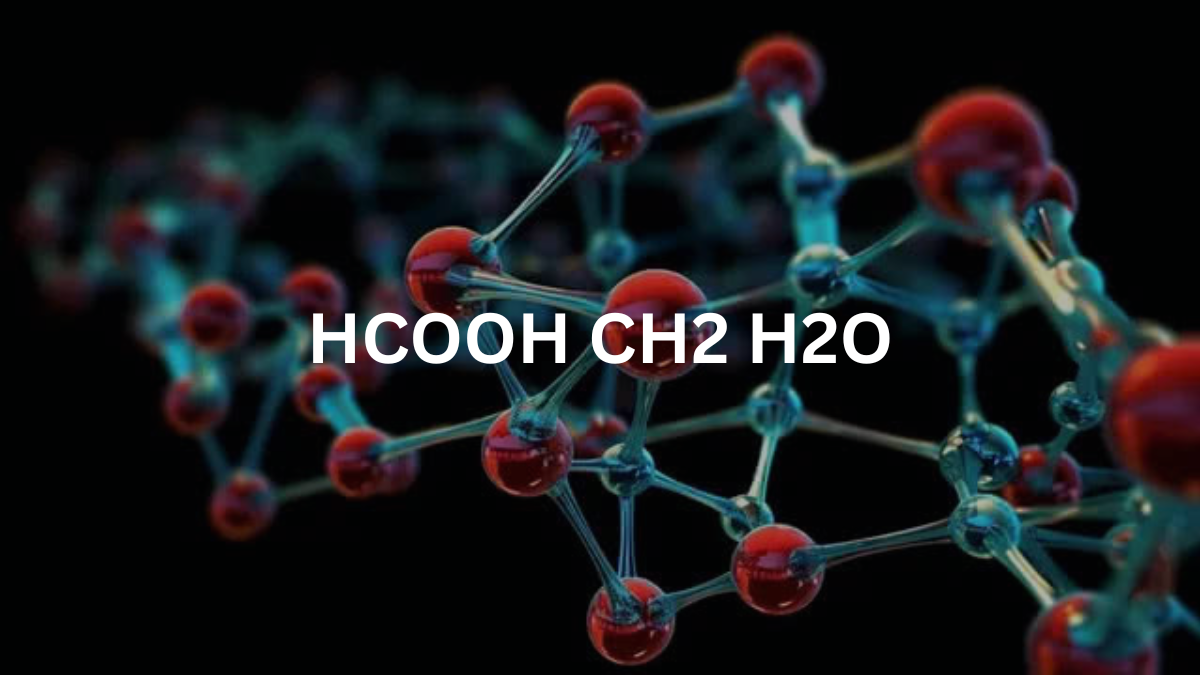
If you’ve stumbled upon the formula HCOOCH CH2 H2O and thought, “Wait… what on Earth is that?”—you’re not alone. At first glance it looks like something straight out of a chemistry textbook. But behind those strange letters lies a fascinating story that connects to the way we fuel our cars, make medicines, design plastics, and maybe even understand the origins of life itself. Let’s unpack this mystery together.
Breaking Down the Formula
So, what exactly is HCOOCH CH2 H2O? Don’t worry—you don’t need a PhD in chemistry to follow along. Let’s take it piece by piece:
-
HCOOCH (methyl formate): This is a type of chemical called an ester. Esters often smell fruity and are used in perfumes and flavorings. Methyl formate specifically is a handy industrial compound—flammable, versatile, and sometimes even considered for fuel.
-
CH2 (methylene group): This little structure is like the Lego block of organic chemistry. It helps build plastics, fuels, and all sorts of carbon-based materials. Even though it seems super simple, it’s the backbone of the modern materials we use every day.
-
H2O (water): Ah yes, our old friend—literally the elixir of life. Water is the medium where a lot of chemical reactions happen, and in this context, it’s not just a solvent but also a participant.
When you see these three written side by side, imagine them as reactive partners on a dance floor, ready to create new combinations that chemists get really excited about.
Why Are Scientists Talking About This Now?
The world is racing to find cleaner fuels and sustainable materials, and that’s where this little chemical trio sneaks onto the stage.
-
Methyl formate (HCOOCH) could be tapped as a cleaner-burning fuel or fuel additive.
-
CH2 groups are crucial for plastics and polymers, meaning they potentially tie into conversations about recyclable or biodegradable materials.
-
And with water in the mix, chemists can explore reactions that don’t depend on toxic organic solvents.
Suddenly, HCOOCH CH2 H2O isn’t just classroom chemistry—it’s an ingredient for tomorrow’s technologies.
Everyday Connections You Didn’t Expect
It’s easy to dismiss chemical formulas as “too abstract,” but here’s how this trifecta touches our daily lives:
-
Fuel for cars and planes: If methyl formate finds its place in biofuel development, what you pump into your tank in a decade could trace its roots back here.
-
Plastic bottles and packaging: CH2 forms the skeleton of polyethylene, the plastic we see everywhere. Researchers are learning how reactions with water or esters change the properties of plastics—maybe even making them greener.
-
Flavors & Fragrances: That fruity scent in your favorite candy? Thank esters like methyl formate. Their solubility in water decides how flavors are blended.
-
Medicine on the shelf: If your pill dissolves when you take it, that’s thanks to ester-water chemistry working properly.
The “Action Scene” in the Lab
Here’s where it gets exciting—if you take methyl formate and let it interact with water, you get a reaction called hydrolysis.
HCOOCH3+H2O→HCOOH+CH3OH
What happens here? Essentially, methyl formate splits into formic acid and methanol. That’s chemistry talk for: one industrial compound creates two others that can be useful in everything from fuels to textiles.
Now add CH2 into the bigger picture. Once chemists weave methylene into the reaction chain, suddenly longer carbon backbones can be formed—structures we need for polymers, fuels, and advanced materials.
It’s like starting with three basic chords and then building a whole symphony.
Green Chemistry Goals
One of the biggest reasons scientists are revisiting compounds like HCOOCH CH2 H2O is sustainability. Water-based reactions mean less dependence on harsh solvents. Methyl formate offers cleaner burning potential compared to fossil fuels. And CH2 opens the door to recyclable or more eco-friendly plastics.
But here’s the honest truth: it’s not all roses. Methyl formate can be toxic at higher concentrations, and handling methylene groups takes serious precautions. The challenge right now is figuring out how to make these processes safe, scalable, and affordable.
What the Experts Are Saying
Dr. Asha Raman, a chemical engineer in Bengaluru, summed it up perfectly: “What excites us most is the simplicity of these molecules. They’re tiny, but their interactions shed light on everything from fuel production to the chemistry that might have existed on early Earth.”
And it’s true. Interestingly, astronomers have even detected methyl formate floating in interstellar dust clouds. Imagine that—molecules tied to our cars, plastics, and perfumes are also hanging out in outer space!
Cool Directions in Research
Here’s a peek at where HCOOCH CH2 H2O is showing up in cutting-edge research today:
-
Biofuels: Developing alternative fuels that are energy-rich but less polluting.
-
Medical chemistry: Crafting water-soluble forms of esters to create better drug delivery systems.
-
Hydrogen storage: Some scientists think methyl formate could help store hydrogen safely for fuel cells.
-
Nanotech & Materials science: Building next-gen polymers where methylene chains are fine-tuned to be stronger, lighter, or biodegradable.
Each of these areas has one thing in common: we’re learning how to make simpler molecules work harder—and cleaner—for us.
Challenges Setting the Pace
But let’s not sugarcoat this. There are still big hurdles ahead:
-
Controlling hydrolysis so it doesn’t produce too many unwanted byproducts.
-
Making sure large-scale processes using methyl formate don’t pose safety risks.
-
Designing catalysts clever enough to handle tricky water-organic reactions efficiently.
-
Keeping costs low enough for industries to actually adopt these methods.
Still, every researcher you’ll speak to is hopeful. After all, 40 years ago ethanol fuel was seen as “fringe science,” and now millions of vehicles run on it. Could methyl formate follow a similar path?
A World Watching
From the United States to India, and Japan to Germany, labs are buzzing. Each nation is tackling green chemistry in its own way, but the crossover focus is clear—water-friendly, cleaner reactions involving esters and hydrocarbons are the future.
And get this: because methyl formate is found in space, astrophysicists think studying it on Earth could one day help us understand how life’s building blocks formed billions of years ago. So yes, this little formula really could help answer some of humanity’s oldest questions.
Wrapping It Up
So the next time you see something as puzzling as HCOOCH CH2 H2O, know this: it’s not just a string of letters. It’s a clue to how chemists are reimagining the building blocks of our world. Whether it’s in your fuel tank, your medicine, or even in the cosmos, this trio of chemicals is stirring up conversations that could reshape industries—and maybe even the way we understand life itself.
Sometimes, the smallest formulas make the biggest difference.